SAS Clinical Online Training

SAS Clinical Online Training
The SAS Clinical Course provides comprehensive training in data analysis and reporting for the pharmaceutical industry. Participants learn essential skills in data management, statistical analysis, and clinical trial reporting using SAS software. Gain expertise in data validation, clinical data integration, and regulatory compliance. Equip yourself with the tools and knowledge to excel in the field of clinical research and make an impact in drug development.
Course Overview
Online SAS training is a course that will open a huge amount of job opportunities. Our organization has hired the best instructors who have a lot of knowledge of the subject. They will provide you with SAS training and make you an expert so that you can handle different positions easily. Theory and practical classes will be arranged for you during the Clinical SAS course and you can get the job of Clinical SAS programmer. A SAS Clinical tutorial will be provided to you during SAS clinical online training to understand the concepts and revising them before taking up the exam.
SAS Clinical Online Training Key features
- Instructors will be available 24/7
- Learn SAS programming in this course
- Get a SAS Clinical tutorial for reference
- Complete clinical SAS certification and get a certificate
- SAS Clinical interview questions will be provided
- Learn analysis of Clinical trials using SAS
- Learn about clinical SAS
Who Should Take SAS Clinical Programming Course?
The course can be taken up by those people who have basic or intermediate knowledge of SAS clinical programming.
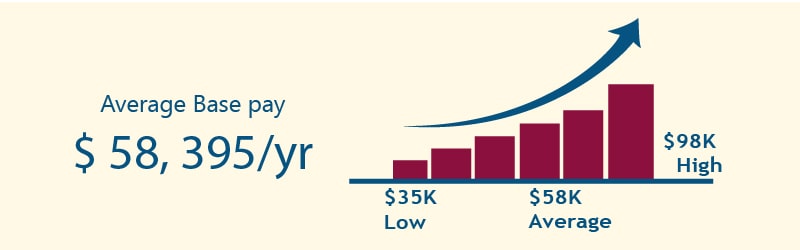
Top Hiring Company

Industry Trends
Course curriculum / Syllabus
- What is clinical research process (phases, key roles, key organizations)?
- Interpret a Statistical Analysis Plan.
- Derive programming requirements from an annotated Case Report Form and a SAP.
- Describe regulatory requirements (International Conference on Harmonization, principles of 21 CFR Part 11, Good Clinical Practices).
- Identifying the classes of clinical trials data (demographic, concomitant medication, baseline, lab, etc.).
- Identify key CDISC principals and terms.
- Describe the purpose and structure of the CDISC SDTM data model.
- Describe the purpose and structure of the CDISC ADaM data model.
- Describe the contents and purpose of define.xml.
- How regulatory requirements are applied to the exported SAS data sets (SAS V5 requirements)?
- Access DICTIONARY Tables using the SQL procedure
- Examine and explore clinical trials input data (missing vs. zero values, find outliers, etc).
- How windowing techniques and categorization are applied to clinical trials data?
- Transpose SAS data sets.
- Apply ‘observation carry forward’ techniques to clinical trials data (BOCF, LOCF, WOCF).
- Calculate ‘change from baseline’ results.
- Obtain counts of events in clinical trials.
- Using SAS procedures for obtaining descriptive statistics for clinical trials data (FREQ, SUMMARY, MEANS, UNIVARIATE).
- How to obtain p-values through for categorical data (2×2 and NxP test for association).
- Use PROC TTEST for obtaining p-values for continuous data (one-sample, paired and two-sample t-tests).
- Create output data sets from statistical procedures.
- Creation and usage of user-defined and automatic macro variables.
- Automate programs by defining and calling macros.
- Usage of system options for debugging macros and display values of macro variables in the SAS log (MPRINT, MLOGIC, SYMBOLGEN, MACROGEN).
- Use PROC REPORT to produce listings and tables for clinical trials reports.
- Use global statements and ODS to produce and augment clinical trials reports.
- Explanation of the principles of programming validation in the clinical trial industry.
- Utilizing the log file for the validation of clinical trial data reporting.
- Usage of programming techniques to validate clinical trial data reporting (MSGLEVEL, PROC COMPARE).
- Identify and Resolve data, syntax and logic errors.
SAS Clinical Online Training FAQ’s:
There is a huge demand or a certified SAS clinical programmer and this course will open a wide variety of opportunities for you.
Yes! They are expert in the field. We hire an instructor after testing them fully. We check their knowledge and then finalize them.
Yes! You will get a video recording for each lecture.
We will arrange tests and you will feel that you are giving the real exam. These tests will include such type of questions that may be asked in the real exam.
Yes! Mock interviews will also be conducted.
Enquire Now
Why QTS INFO
Best Virtual training classrooms for IT aspirants
Real time curriculum with job oriented training.
Around the clock assistance
We are eager to solve your queries 24*7 with help of our expert faculty.
Flexible Timings
Choose your schedule as per your convenience. No need to delay your work
Mock projects
Real world project samples for practical sessions